 |
Polymorphism & Seeding
I. Discussion |
 |
Polymorphism & Seeding
I. Discussion |
Discussion
As we have seen powder diffraction techniques are vital in phase identification in patents, and this is nowhere more important than in situations where a compound can form more than one crystal structure, i.e., where the compound exhibits polymorphism. The possibility of crystallising these different phases from one solution makes for more intrigue, and lawyers without scientific training sometimes have difficulty in grasping the consequences.
Polymorphism
"Where relevant the presence of polymorphic forms and the methods of determination and control should be discussed, or their absence confirmed." Medicines Act 1968, Guidance Notes on Applications for Product Licences (MAL 2).
Consequences of Polymorphism
Chemically, polymorphs may appear the same (i.e. they may appear identical by liquid phase n.m.r. or any elemental analysis for instance) but they often have different physical properties. For instance, their melting points may well be different. Patents for drugs may need to specify a particular polymorph or polymorphs, because of the critical importance of rates of dissolution. In industrial terms the shelf-lives of different polymorphs can differ, as can their grindability, rheological properties, or hygroscopicity.
Pseudo-polymorphism is a less precise term. In the context of pharmaceutical patents it may be taken to include different types and amounts of solvents of crystallisation, so crystalline zytican monohydrate and crystalline zytican hemi-hydrate are different pseudo-polymorphs. I've even come across "pseudo-morph" used, but this has a completely different meaning in mineralogy.
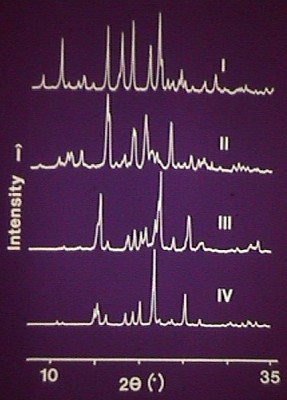
Powder diffraction patterns of four forms of sulphathiazole were published in the Journal of Pharmaceutical Sciences, 1989, 78, 337-342, by Jamshed Anwar et al., Polymorphism of Sulphathiazole. This paper discusses grinding and preferred orientation, identification of polymorphs by powder diffraction, the use of a Gandolfi camera for getting a powder pattern from single crystals and the computer simulation of powder patterns from known structures as newly discovered polymorphs. Other methods were also used to support the characterisation.
How is Polymorphism Detected ?
Clearly a method which is sensitive to crystal structure is needed. Anything involving dissolution, destruction of the unit cell or similar like liquid phase n.m.r., mass spectroscopy or most methods of elemental analysis can be of no use at all. Methods which mainly probe bonding may not be very sensitive to polymorphism, since often there are only minor changes to intermolecular hydrogen bonding. Differential Scanning Calorimetry and hot stage microscopy can assist in probing for polymorphism. However, crystallographic techniques will clearly be the most sensitive, since even a very subtle change in inter-molecular interaction which produces a new polymorph can result in significant differences in the powder diffraction pattern.
Powder diffraction data collected under non-ambient conditions (e.g. at high temperature) can often be very useful, enabling one to probe transformations, stabilities and even kinetics of transformation.
Seeding
The so-called "disappearing polymorphs" phenomenon sometimes occurs when crystals are grown from seed. The problems entailed by this effect have impacted on several major pharmaceutical patent cases, so a brief description of it is given here.
Some of you may remember growing copper sulphate or alum crystals at school by putting a "seed" crystal into a supersaturated solution (for instance by hanging one on a string). Nucleation and growth are quite distinct, however, and much effort can go into the production of a seed crystal of a new phase.
Imagine the bench chemist with one of dozens of possibly interesting compounds working away to try to crystallize that compound, which is often quite difficult in the first instance. Finding the right set of conditions for a nucleation can take a long time. Eventually they get crystals and using seeds from the first crop the method is scaled up for production of test batches and a patent is prosecuted, perhaps with an powder diffraction pattern to identify the crystal phase, or perhaps not; the simple molecular structure from n.m.r. and an infrared pattern might be considered sufficient to characterise the new phase. At all stages the new samples are seeded with previous crystals: that polymorph dominates (in fact at this stage polymorphism is not even a question). The situation is represented by the diagram below. The nucleation barrier that must be overcome to proceed from a super-saturate solution to growth can be circumvented by seeding. This process is represented by the dotted path.
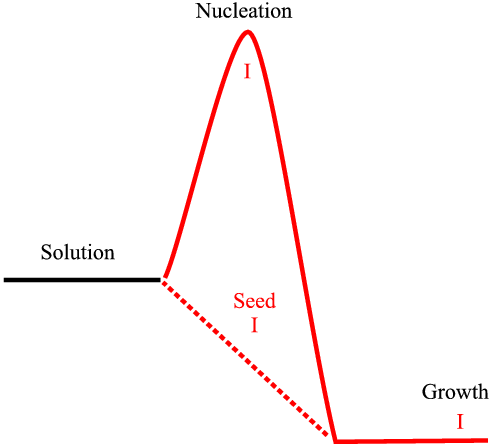
If all goes well the compound might prove to be advantageous and be tested further. Perhaps the question of polymorphism arises and efforts are made to re-crystallize, perhaps from different solvents, so later, (perhaps many years later) a new polymorph is found. If this happens to be faster growing (even though it might have been more difficult to nucleate) then it will come to dominate. If a polymorphic form were easier to spontaneously nucleate we may have expected it to nucleate first. If another polymorph were more difficult to nucleate and slower to grow we might never expect to find it - the seeding with the original polymorph would dominate.
However, in the more common case, where the second form is more difficult to nucleate but grows more quickly, generally it is the second polymorph which is patented, very probably along with its powder diffraction pattern. Now the twist is that if someone with seeds of the second polymorph were to repeat the method outlined in the first patent, they might well end up with the second polymorph and not the first one. This can baffle people who do not understand crystallography; a lawyer may well argue that either the first patent is invalid (it is not disclosing - if I repeat the experiment I do not get what is claimed) or that the second patent is invalid because it is not novel (because the previous patent covers the same material, and everyone knows you can't patent something twice).
Of course we know that repeating the procedure given in the first patent, but with seeds of the second polymorph (and these seeds may be extremely small, perhaps just a few unit cells in volume and may be floating in the air) is NOT the same experiment. The situation is worse when polymorph II grows much quicker than polymorph I, even though polymorph I may well spontaneously nucleate more quickly than polymorph II.

This situation is illustrated in the diagram above. The original form I is still present as before. However, the crystal growth is taking place under conditions favouring the nucleation of form II. If a supersaturated solution is then seeded with a mixture of forms I and II much more of the form II will crystallise, and if that product is then used to crystallise the next batch the seed will be mostly form II and the subsequent batch will be overwhelmingly form II as so on. Hence the riddle of the disappearing polymorphs: form I will become less and less common. Professor Jack Dunitz gave the 1999 Bragg lecture at the I.U.Cr. meeting in Glasgow along this very theme. Two examples of polymorphism and seeding follow in the next page.
Footnote: There is an interesting short letter to the editor in the Journal of Applied Crystallography, 1975, 8, 342, by Woodward and McCrone entitled Unusual crystallization behaviour which begins: "There are many instances, some reported in the literature, and many others not, of crystalline compounds behaving respectably for many months or years until nucleation of a more stable form."
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Simon Jacques
Stephen Tarling |