The
electromagnetic spectrum no. 24 in a
series - dedicated to Chris Cronin
The electromagnetic spectrum is the physicist’s stained glass window on the physical universe. The beauty of the individual colours combine to form a complete picture of nature ranging from the spatial information that the long wavelength radio waves contain to the uncertainty of the energetic g rays. From the broad reddish end to the fine violet at the other extreme, in between lie all the colours of the rainbow. The colours represent size in one picture of space and in a complementary view the energy associated with that region. This analogy is very good in helping to appreciate the reciprocal relationship between the wavelength of electromagnetic waves and the energy they possess.
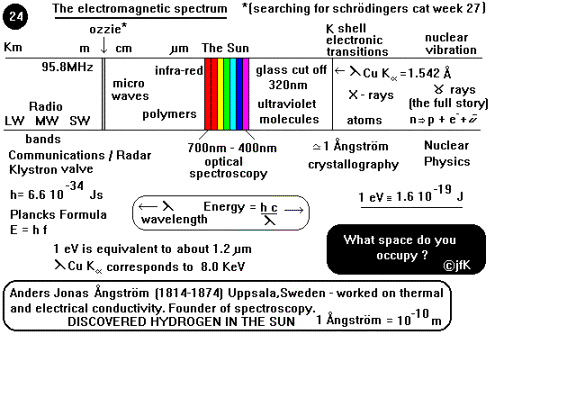
At the long end radio waves
have wavelengths of several kilometres with low frequencies, while the shortest
wavelength most penetrating g rays l » 10 -16m, with
such small distances involved it is customary to express the photon energy
instead (Energy is proportional to frequency). The radio waves are used in
communication (TV as well as the customary car radio) and navigation by radar
(AMDS week 29), they are produced by klystron valves. Radio sources exist in
pulsars at the core of some of the most mysterious objects in the galaxy. At
home in the kitchen are the familiar centimetre waves known as microwaves (mm) useful in heating polar
molecules (have you ever wondered why foods containing salt and water cook
fastest?).
The infra red rays (i.r.)
were discovered in 1800 when William Herschel placed a thermometer beyond the
red end of the visible spectrum and noticed a temperature rise, they are now
commonly used in the heat treatment of professional sports injuries. The
optical part of the spectrum that our senses see spans the range from 700 nm to
400 nm and gives us in that window the information that we process in order to
make sense of the interaction of the rest of the spectrum with matter. The
ultraviolet part of the spectrum is now widely used in the high street to
detect forgery (do you know where?) and incidentally cannot pass through glass.
The K shell electronic
transitions within the atomic orbital give rise to arguably the most important
part of the spectrum particularly as far as scientists are concerned the region
discovered by Wilhelm Röntgen in 1895 and known as x-rays. The wavelength of
the Copper atom K shell transition lCuKa = 1.542 Å and is of the
same order of magnitude as the size of an atom thereby allowing diffraction by
the planes of atoms in crystals which gives rise to the science of x-ray
crystallography. When the nucleus of an atom is in an excited state in order to
relax it emits a high-energy g ray with
virtually no mass and no charge, which easily penetrates several cm of lead.
Nuclear medicine relies on the production of radionuclides for example from a
Mo generator (AMDS week 32), which produces the isotope Technetium 99m.
This decays with the emission of a 144 keV photon with a half life of 6 hours
which is used in radio-labelling many compounds for g camera imaging e.g. brain,
lungs, liver, spleen, skeleton, kidneys and thyroid.
The
wave equation
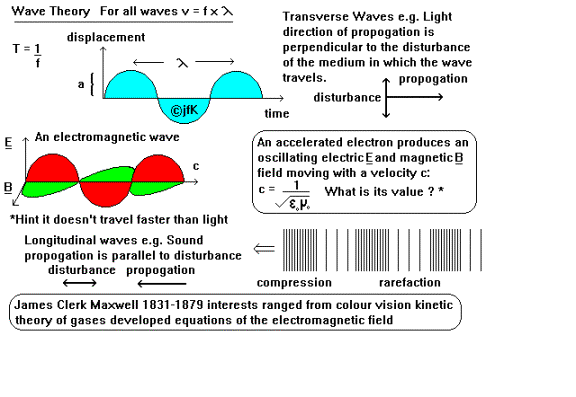
All waves whether transverse
or longitudinal (see diagram) are described by three parameters their velocity
of propagation, wavelength and frequency. A complete cycle of the wave is known
as its wavelength (symbol l, unit metre) and
the time taken to complete it is known as the periodic time T, a useful measure
is 1 / T which is known as frequency (symbol f unit s-1 known as
Hertz). For any wave it is true:
v (
m s-1 ) = f ( s-1 ) x l ( m ) v
= f x
l
For an electromagnetic wave
the velocity of light c is constant c = 3 x 108 m s-1 and
the frequency is more often written as n (nu) so: c = n x l
Using the Planck equation
(AMDS27): E = h x n and substituting for n n = c / l
gives us a relationship
between Energy and wavelength:
|
E
= h x c l |
Problems
1. Calculate the wavelength of the radio station
Capital transmitting on 95.8 MHz FM.
2. What is the frequency of the AM transmission of
Capital Radio which broadcasts on a wavelength of 194 m ?
3. What is the range of the optical spectrum in
electron Volts ?
4. What is the
wavelength of a 30keV x-ray photon ?
5. Calculate
the wavelength of the microwaves used in a food processing if their frequency
is 2.45 GHz ?