The Astrophysical Nature of Silicon Carbide
======================================================================================
Silicon Carbide - Older than the Stars
On the origins of Moissanite
Silicon carbide (SiC) is even older than our solar
system having wandered through the Milky Way for billions of years as stardust
that was generated in the atmospheres of carbon rich red giant stars and from
supernova remnants. The gravitational coalescence of our solar system trapped
micron size silicon carbide grains in the meteorites that were forming from the
accretion of the debris in clouds of interstellar gas. The discovery of these
refractory grains has led to the unlocking of their cosmic chemical memory.
Primitive meteorites contain minute amounts of
pristine interstellar material, in particular SiC (Pillinger et al. 1993), that
point to the nuclear and chemical processes that occur in stars. Anders and
Zinner (1993) have reviewed the subject and conclude that the grain size and
abundance of silicon carbide shows the signature of the s-process originating
from red giant carbon (AGB) stars of 1-3 solar masses with an age > 109
a. [s-process nucleosynthesis occurs when neutrons are captured at a slow rate
relative to β-decay rates which occurs extensively during the Asymptotic
Giant Branch phase of stellar evolution, shown graphically on the
Hertzprung-Russell diagram Fig. 1.1]
Recent analysis of SiC grains found in the Murchison
carbonaceous chondrite by Hoppe et al. (1994) has revealed that these starry
messengers contain anomalous isotopic ratios of carbon and silicon. Clayton
(1997) and Timmes and Clayton (1996) have discussed the galactic nature of
these primitive pre-solar grains in terms of the ratios of 29Si/28Si
and 30Si/28Si found. These are more abundant than in our
sun indicating an origin from outside the solar system and closer to the
galactic centre. Isotopic analysis of meteoritic silicon carbide pre-empts the
information that may be recorded in the grains and is now offering a new and
exciting tool for exploring the structure and evolution of our galaxy.
However the indications are that the meteoritic
silicon carbide data point to the presence of the cubic-β polymorph rather
than the predominant α-hexagonal polymorph as found in laboratory
experiments. Reasons for this anomaly are not clear (Kelly 2001). There has
been considerable debate in the astronomical literature (Speck 1998) over the
discrepancy between the meteoritic and astronomical identifications of the SiC
type. The 11.3 μm infrared SiC emission band from the dust envelopes
surrounding C rich AGB stars matches the α type, while the meteoritic
presolar grains of SiC have found to be of the β form.
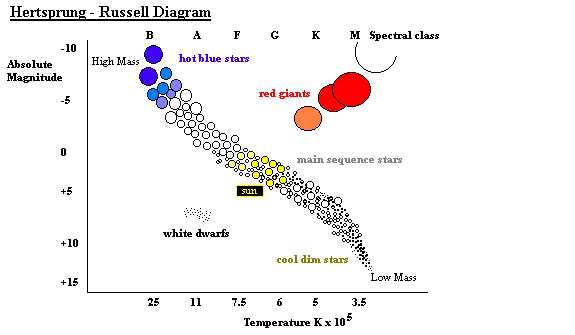
Figure 1.1 A representation of the
Hertzprung-Russell diagram of stellar evolution in which the bightness of a
star is plotted against its colour [Absolute magnitude (5 magnitudes correspond
to a difference of 100 in the measured light intensity of a star) versus its
temperature (K)]. A cluster of cool bright stars to the top right of the
diagram indicate that they are very large, these red giants possess an outer
gas mantle surrounding an inert He core formed once H is exhausted from
thermonuclear fusion. Successive core burning produces the heavier elements in
AGB stars so named because they evolve along a track parallel to the RGB stars.
Isotopic
analysis of silicon carbide grains from meteorites (Anders and Zinner 1993)
indicate characteristics of slow neutron capture reminiscent of AGB stars.
These form to the right of the main sequence stars above, on a horizontal and
are part of the red giant branch (RGB). Astronomical observations of the dust
envelopes of these stars also indicate the presence of silicon carbide.
Notwithstanding this, previously an iron meteorite
from a terrestrial impact crater at Diablo Canyon in Arizona was chemically
analysed by Henri Moissan (1905) and found to contain hexagonal platelets of silicon
carbide. This was the first observation of the natural mineral Moissanite (SiC), although its synthetic
preparation and properties had been described earlier by the same author
(Moissan 1893).
Initial manufacture of carborundum
Far from this heavenly scenario Eugene G Acheson had
in fact already produced the first “artificial crystalline carbonaceous
material” as a substitute for diamonds which he described in his 1892
resistance furnace patent. He heated coke and silica together to make a substance
that “may be designated under the general term Carborundum” describing the new compound “hitherto unknown to
chemistry as represented by the formula SiC and that it may be known as
silicide of carbon or carbide of silicon”. (The term was coined from the synthesis
of “carbon” and “corundum”(Al2 O3) indicating its
hardness to lie between that of the two). This paved the way for the
large-scale production of silicon carbide for the abrasives industry, its main
commercial use today. However the idea that a silicon-carbon bond might in fact
exist in nature was first proposed by the Swedish chemist Jöns Jacob Berzelius
as early as 1824, (Berzelius 1824).
