©ICDD 1967. Used with permission from the International Centre for Diffraction Data.
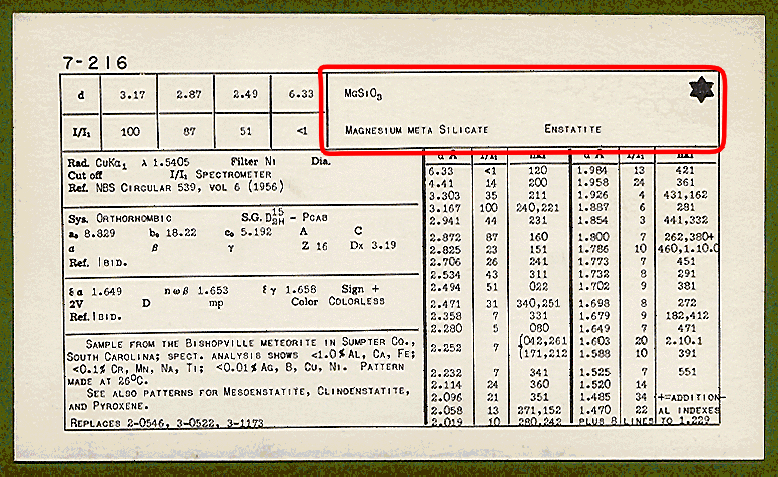
The section of the card outlined in red shows a box containing the chemical formula, chemical name, and mineral or common name. The star (*) in the top right-hand corner indicates that the data is of the highest quality.

©ICDD 1967. Used with permission from the International Centre for Diffraction Data.
![]() The nomenclature of the chemical names for the material identification
follows, in general, the 1957 IUPAC Nomenclature of Inorganic Chemistry:
The nomenclature of the chemical names for the material identification
follows, in general, the 1957 IUPAC Nomenclature of Inorganic Chemistry:
Cations are ordered according to group number within the periodic table and are followed by anions, e.g. Sodium Magnesium Iron Sulfate and not Magnesium Sodium Iron Sulfate.
| © Copyright 1997-2006. Birkbeck College, University of London. | Author(s): Jeremy Karl Cockcroft |