 |
Combined Techniques |
 |
Combined Techniques |
Combined Techniques: Don't Put All Your Eggs Into One Basket!
One single technique rarely gives the full picture of what is going on in a complex process; in-situ diffraction, although powerful, usually needs to be complemented by other techniques. For this reason many diffraction experimenters have got into the business of designing, building and operating what we call multi-technique stations or instruments; that is stations/instruments where two or more in-situ techniques are applied simultaneously to the same sample. Such combinations might for example be:
The authors of this section will express a personal view here. It is that combined technique instruments are necessary for following processes with exact registration but single technique instruments are also necessary to optimise methodology. By this we mean that in the process of building a multi-technique instrument, compromises will inevitably be made that render each technique less good than if the technique were practised on its own. However running a process twice means that you are never quite sure if you have really run the experiment equally on both instruments. So the philosophy that these authors would propose is that the techniques should be run both separately and simultaneously thereby getting the best of both worlds.
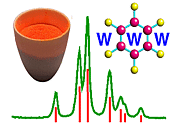
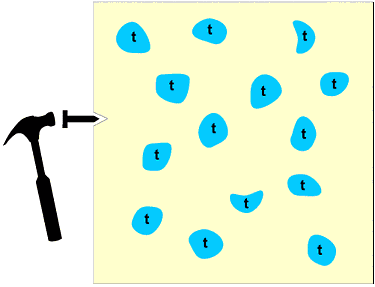
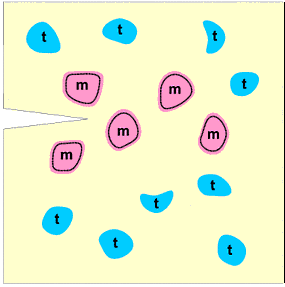
Again there are endless numbers of examples, worldwide, that one could give so just one illustrative example will be taken here from the in-house research of the authors. The topic is zirconia: synthesis and transformations. Zirconia is an important ceramic material which finds applications in diverse areas such as oxygen sensors, catalyst support, thermal barriers and mechanical functions. Monoclinic zirconia particles can be made meta-stable at room temperature and this feature is exploited to impart fracture resistance to ceramic devices such as high temperature dies, cutting edges, turbine blades and engine components: the monoclinic to tetragonal transformation is tailored so as to "blunt" the progress of threatening micro-cracks within a ceramic composite as illustrated in the following schematic diagram:
 |
 | |
Three examples of the uses of zirconia, illustrating respectively its high temperature resistance, aesthetic qualities and mechanical durability, are shown here (131 kB).
The final stages in the synthesis of zirconia include the calcining of zirconium hydroxide to about 1000°C and subsequent cooling back to room temperature, as indicated in the following idealised sequence:
| 20°C | 450-600°C | 1000°C | 20°C | |||
| Zr(OH)4 | → | ZrO2 + 2H2O(gas) | → | ZrO2 | → | ZrO2 |
| amorphous | tetragonal | tetragonal | monoclinic |
But this is a simplified picture; for example the precursor is not really Zr(OH)4 but a mixed hydroxide, oxide and hydrate. The process above has been studied using a combination of powder diffraction, EXAFS and neutron scattering and is each discussed in turn.
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Simon Jacques Martin Vickers |