 |
The Value of Neutrons
II. Thermal Decomposition |
 |
The Value of Neutrons
II. Thermal Decomposition |
Thermal Decomposition of Cobalt Acetate Hydrate
This study by Grimes and Fitch using the ILL neutron source (J. Mater. Chem. 1, 461 (1991)) is a nice example of how one can extract additional information concerning specific parts of a structure by selective deuteration. The study was of the decomposition of cobalt acetate hydrate (Co(CH3CO2)2.4H2O) into cobalt oxide, CoO, as it is heated up from room temperature to 600°C. The initial starting powder could be made in 4 alternative forms:
| 1. | (Co(CH3CO2)2.4H2O) | no deuteration (i.e. hydrogenated form) | ||
| 2. | (Co(CD3CO2)2.4H2O) | deuterated acetate groups | ||
| 3. | (Co(CH3CO2)2.4D2O) | deuterated water | ||
| 4. | (Co(CD3CO2)2.4D2O) | fully deuterated |
The in-situ neutron diffraction patterns obtained in these 4 cases were very different as seen in the next figure in which, for visual comparison purposes, the maximum heights of each sequence have been made similar:
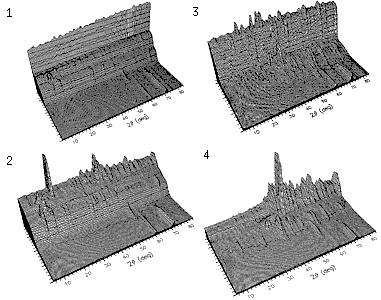 |
click on image to enlarge (186 kB) |
The large background in three of these is due to the presence of hydrogen and therefore the drop in these backgrounds as the temperature rises (i.e. the step-like appearance) represents the loss of hydrogens from either the acetate group, CH3CO2, or the water, H2O, or both. Even by just looking at these pattern sequences one can make some qualitative observations regarding the decomposition process, for example consider:
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Simon Jacques Martin Vickers |